This post is also available in: Persian Armenian
3D printing, or Additive Manufacturing (AM), has transformed modern manufacturing over the past few decades. This technology enables the production of parts with highly complex geometries, reduced material waste, shorter lead times, lower development costs, and rapid prototyping as well as on-demand, one-off components.
Although 3D printing can be performed with a wide range of materials—including polymers, resins, and metals—metal additive manufacturing requires significantly higher levels of control and process stability due to the physical and metallurgical characteristics of metals.
In metal AM, metallic powders are selectively melted and consolidated layer by layer. This process takes place at very high temperatures and in a highly sensitive environment. The presence of oxygen or moisture can lead to:
- oxidation of the molten metal,
- deterioration of surface quality,
- and degradation of the mechanical properties of the finished part.
For this reason, maintaining a controlled, inert atmosphere is critical.
Argon, as a noble and chemically inert gas, plays a central role in creating and maintaining this controlled environment. It not only suppresses unwanted chemical reactions, but also helps preserve the intrinsic properties of the printed metal and ensures consistent, high-quality output. In addition, the use of argon is essential for protecting operators and equipment when processing highly reactive metal powders.
Argon: A Noble Gas and Its Key Properties
Argon is a noble gas in the periodic table, widely used as a shielding and protective gas in sensitive industrial processes due to its colorless, odorless, and chemically inert nature.
Physical and Chemical Properties of Argon
Colorless and odorless
Argon has no color or odor and, under normal conditions, does not readily react or mix chemically with its surroundings.
Chemical inertness
Argon has extremely low reactivity. It does not react with oxygen, water, or most metals at ambient temperature. This makes it ideal wherever a neutral, non-reactive atmosphere is required.
Low boiling and melting points
The boiling point of argon is approximately −185.8 °C, and its melting point is around −189.3 °C, enabling its use in a wide range of industrial temperature conditions.
Purity grades
Industrial argon is typically supplied at a purity of 99.9%, while high-purity grades (99.995% and above) are available for sensitive applications. The higher the gas purity, the more suitable it is for printing reactive metals such as titanium and specialty alloys.
These properties make argon a key gas wherever tight atmosphere control is required, especially in metal additive manufacturing.
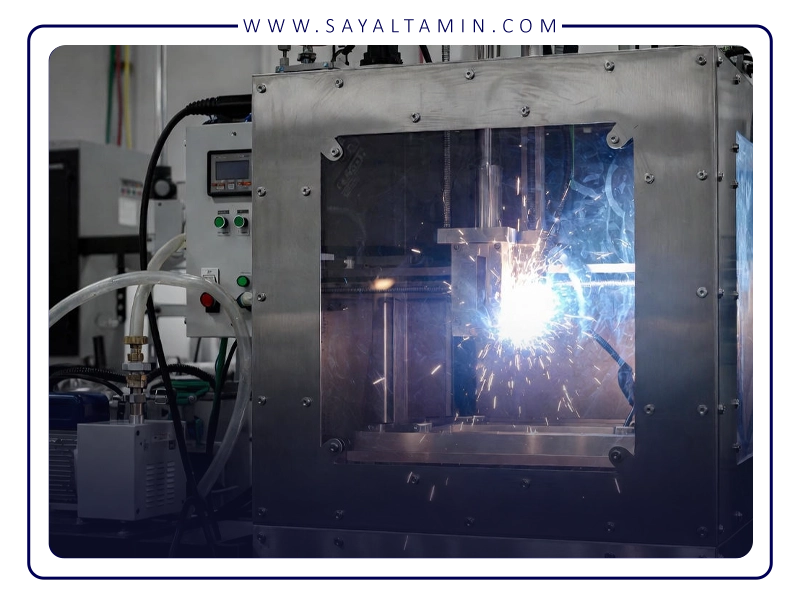
The Role of Argon in Metal 3D Printing
Metal 3D printing is a complex, multi-stage process in which metallic powders are melted using a laser, electron beam, or other energy source. The process involves extremely high temperatures and rapid thermal cycles, which significantly increase the tendency of metals to react with oxygen and moisture in the environment.
Even very low levels of oxygen can lead to the formation of oxide films and inclusions, which adversely affect both surface quality and the mechanical performance of the part—such as strength, toughness, and hardness.

Preventing Oxidation
Molten metals are highly reactive. Light and reactive metals such as titanium and nickel-based alloys are especially prone to oxidation, even at trace oxygen levels. Oxide formation can cause:
- poor interlayer bonding,
- internal porosity,
- and weak mechanical zones within the part.
By establishing an inert argon atmosphere, contact between the molten metal and oxygen is minimized. This stable environment allows the melt pool to form and solidify cleanly, resulting in:
- uniform surfaces,
- low contamination,
- and high-quality, consistent layers.
Preserving Mechanical Properties
Argon shielding not only prevents surface oxidation, it also helps avoid internal reactions and microstructural degradation. As a result, key mechanical properties—such as tensile strength, hardness, toughness, and fatigue resistance—can be maintained throughout the entire volume of the part.
Without a proper argon atmosphere, internal stresses and thermal gradients can promote:
- microcracking,
- weak interfaces between layers,
- or localized embrittlement,
which can subsequently cause unexpected failure in demanding industrial applications.
Safety When Working with Reactive Metals
Some metals used in AM, including titanium, nickel superalloys, and aluminum, are capable of intense exothermic reactions or combustion when exposed to high oxygen concentrations at elevated temperatures—especially when present as fine powders.
In such cases, argon is used to displace oxygen and reduce the risk of:
- flash fires,
- dust explosions,
- and uncontrolled reactions in the build chamber or powder handling system.
Thus, argon contributes not only to product quality, but also to operator safety and process reliability.
Use of Argon Across the Metal 3D Printing Workflow
Powder Production and Storage
The quality of metal 3D printed parts is directly linked to the quality of the powder feedstock. Ideal characteristics include:
- spherical particle morphology,
- narrow and controlled particle size distribution,
- and high chemical purity.
In gas atomization processes, argon is commonly used as the atomizing and protective gas. This prevents the molten metal droplets from contacting oxygen and moisture during solidification. For reactive metals, even brief exposure to air can:
- form oxide layers,
- alter surface chemistry,
- and degrade powder flowability and packing behavior.
Storing powders under argon—whether in sealed containers or argon-purged cabinets—further prevents moisture uptake and undesirable chemical reactions, extending powder shelf life and maintaining consistent print quality.
Build Chamber (Print Environment)
During printing, the build chamber is first purged with argon to remove air (oxygen and moisture). The chamber is then maintained at a low oxygen concentration—typically well below 1000 ppm, and often much lower for reactive alloys.
Benefits of argon in the build chamber include:
- prevention of oxidation during melting and solidification,
- formation of clean, metallurgically sound layers,
- reduction of spatters and oxide-related defects,
- and assistance in achieving more uniform heat distribution.
By mitigating localized hot and cold spots, argon helps minimize:
- warping and distortion,
- unwanted residual stresses,
- and thermally induced cracking.
The result is a part with smooth surfaces, high dimensional accuracy, and uniform mechanical properties.
Heat Treatment and Post-Processing
After printing, metal parts often undergo heat treatment or hot isostatic pressing (HIP) to:
- relieve internal stresses,
- increase density,
- refine microstructure,
- and improve mechanical performance.
Furnaces used for these post-processing steps are typically filled with argon to prevent the parts from reacting with oxygen or moisture at elevated temperatures.
Argon in post-processing:
- preserves surface appearance and prevents scale formation,
- maintains mechanical properties by avoiding additional oxidation,
- enhances safety by reducing the risk of ignition when treating reactive alloys.
This ensures that the benefits gained during the build process are not lost during subsequent thermal cycles.
Metals and Alloys Commonly Printed Under Argon
Metal AM is used for a wide range of metals and alloys, each with its own sensitivity to oxidation and reactive gases. The choice of atmosphere—often argon or argon mixtures—is therefore critical.
Stainless Steels
Stainless steels are among the most common materials in metal AM. At high temperatures, however, they are still susceptible to oxidation and unwanted surface films, which can lead to:
- porosity,
- lack of fusion,
- reduced mechanical strength.
An argon atmosphere:
- keeps the surface of stainless steels clean and uniform,
- improves interlayer bonding,
- and enables the production of parts with high tensile strength and hardness.
Titanium Alloys
Titanium and its alloys are widely used in aerospace, medical, and automotive applications due to their high strength-to-weight ratio and biocompatibility. However, titanium is extremely reactive at elevated temperatures and readily forms oxides and nitrides.
Printing titanium under argon:
- prevents oxide and nitride layer formation,
- preserves ductility and fatigue life,
- and ensures both process safety and product reliability.

Aluminum and Copper
Aluminum and copper are lightweight, high-conductivity metals that are challenging to print due to:
- high thermal conductivity,
- and strong tendency to oxidize.
Argon helps stabilize the thermal environment in the build chamber and protects the melt pool from oxidation, enabling:
- better dimensional accuracy,
- reduced porosity,
- and smoother as-printed surfaces.
Nickel Alloys and Superalloys
Nickel-based superalloys are critical for aerospace, power generation, and high-temperature applications. They are extremely sensitive to oxygen during melting and solidification; even small amounts of oxidation can:
- change alloy chemistry,
- degrade creep and fatigue properties,
- and limit service life.
Using high-purity argon during printing and post-processing ensures that these alloys are produced with:
- minimal contamination,
- controlled microstructure,
- and optimal mechanical performance.
Benefits of Using Argon in Metal 3D Printing
The use of argon in metal AM improves not only part quality, but also the overall efficiency and safety of the process.
Improved Product Quality
Argon reduces:
- surface defects,
- internal porosity,
- and interlayer discontinuities.
Consequently, printed parts exhibit:
- smoother surfaces,
- higher structural integrity,
- and greater reliability—especially critical in aerospace, medical, and oil & gas applications.
Enhanced Accuracy and Consistency
By promoting uniform heat distribution and stable process conditions, argon helps maintain:
- consistent layer thickness,
- precise geometries,
- and tight dimensional tolerances.
This alignment between printed parts and their original CAD models is essential for high-precision, safety-critical components.
Reduced Scrap and Lower Costs
With fewer defects and less rework, argon contributes to:
- lower scrap rates,
- reduced need for corrective machining or reprinting,
- and overall savings in material, energy, and labor.
Over time, this can significantly improve the economic viability of metal AM.
Increased Process Safety
By creating an inert environment, argon reduces the risk of:
- unwanted reactions with reactive metal powders,
- fires,
- and dust explosions.
This is particularly important when handling and printing metals like titanium and aluminum, where powder ignition can have severe consequences.
Safety Considerations and Potential Hazards
Despite its chemical inertness, argon must be handled with care.

Asphyxiation Hazard
Argon displaces oxygen in confined spaces. In the event of a leak, oxygen levels can drop without any visual or olfactory warning, as argon is colorless and odorless. This can lead to asphyxiation.
Adequate Ventilation
All printing rooms, post-processing areas, and gas-handling spaces must be equipped with proper ventilation systems to quickly remove any leaked argon and maintain safe oxygen levels.
Cylinder Storage and Handling
Argon cylinders should be:
- stored upright and secured,
- kept away from heat sources,
- inspected regularly for valve integrity and leaks.
Transport and handling should follow applicable safety standards to minimize both physical hazards and gas release.
Personnel Training
Operators must be trained to:
- recognize the hazards associated with inert gases,
- respond to leaks and alarm conditions,
- use personal protective equipment correctly,
- and identify early signs of oxygen deficiency.
Comprehensive training is a key component of safe argon use in AM environments.
Technical Challenges and Practical Considerations

Cost of High-Purity Gas
High-purity argon is more expensive than standard industrial gases. Selecting the appropriate purity level for each material and application—and evaluating the return on investment—are important steps in process design and cost optimization.
Equipment Requirements
Argon alone is not sufficient; the AM system must be equipped with:
- sealed build chambers,
- pressure and flow control systems,
- oxygen sensors,
- and suitable ventilation and exhaust systems.
These components ensure that the process atmosphere is properly controlled throughout printing and post-processing.
Training and Operational Discipline
Insufficient training and lack of procedural discipline can lead to:
- improper gas usage,
- safety incidents,
- and suboptimal part quality.
Standard operating procedures and regular refresher training are crucial.
Selecting the Right Gas for Each Metal
Each metal and alloy exhibits different reactivity and process behavior. In some cases, argon mixtures with helium or nitrogen may offer improved heat transfer, reduced residual stress, or better surface properties at lower cost. Optimizing gas selection is therefore part of the broader process engineering strategy.
Future Directions and Innovations in Argon Use
Argon Mixtures with Other Gases
For highly sensitive alloys, combining argon with helium or nitrogen can improve:
- thermal conductivity in the build chamber,
- mitigation of hot and cold spots,
- and reduction of internal stresses.
Such gas mixtures are particularly attractive for aerospace, medical, and superalloy applications, where even minor defects can critically impact performance.
Next-Generation Metal AM
As metal AM advances toward micron-scale precision, lattice structures, and functionally graded materials, the need for tightly controlled inert atmospheres becomes even more important.
Argon-based environments enable:
- precise control of melt pool dynamics,
- uniform layer deposition,
- and the production of complex internal structures with minimal defects.
These developments support the fabrication of parts with exceptional mechanical properties, dimensional accuracy, and long service life.
Impact on Future Industries
- Medical: customized implants and prosthetics with complex, patient-specific geometries and biocompatible materials.
- Aerospace and space: lightweight, high-strength components that improve performance and reduce fuel consumption.
- Automotive and motorsport: optimized, weight-reduced parts for higher efficiency and performance.
- Energy and oil & gas: corrosion-resistant, high-pressure components manufactured for extreme environments.
In all these cases, argon plays a decisive role in enabling reliable, high-performance parts.
As a noble and inert gas, argon is indispensable in metal 3D printing. By creating and maintaining a protective atmosphere, argon:
- prevents oxidation of molten and solidified metals,
- preserves surface finish and mechanical properties,
- supports dimensional accuracy and structural integrity,
- and enables the safe processing of highly reactive metal powders.
From powder production and storage, through the printing process itself, to heat treatment and final post-processing, argon contributes to:
- reduced defects,
- improved repeatability,
- and parts that meet demanding industrial standards.
Argon also makes it possible to print reactive metals such as titanium, nickel alloys, and superalloys safely and reliably, which is critical for advanced applications in aerospace, medicine, energy, and automotive industries.
In summary, argon not only enhances the quality and performance of 3D-printed metal parts, but also enables safe, precise, and sustainable production of complex components. It is a key driver in the continued evolution of additive manufacturing and in the broader industrial shift toward high-value, digitally driven production technologies.
————————————————–
References
1. Air Products. Metal Additive Manufacturing and Gas Applications. Available at: https://www.airproducts.com.tw/-/media/files/en/330/330-19-001-us-additive-manufacturing-and-gas-applications-technology.pdf
2. 3DPrint.com. How Argon and Nitrogen Shielding Gases Affect 3D Printed Stainless Steel. Available at: https://3dprint.com
3. Tebianian, M., et al. (2023). A Review of the Metal Additive Manufacturing Processes. Materials, 16(24), 7514. Available at: https://www.mdpi.com