This post is also available in: Persian Armenian
Introduction
Airbags are one of the most critical safety technologies in modern vehicles. They deploy in a fraction of a second, protecting occupants from severe impacts to the dashboard, steering wheel, and other vehicle components. The optimal performance of airbags is directly dependent on the gases used in them.
In the past, chemicals such as sodium azide were used to produce the gas. However, due to safety and environmental concerns, the use of inert, stable gases such as nitrogen and argon has increased in recent years. The combination of these two gases significantly increases the speed, safety, and durability of airbags.
This article provides a scientific and practical review of the characteristics, benefits, production process, and use of argon and nitrogen mixtures in airbags and is useful for automobile manufacturers, gas suppliers, and automotive safety enthusiasts.
Airbag performance and gas requirements
Airbags are designed to fully deploy within 20 to 30 milliseconds of impact. This rapid performance requires gases that have both a high expansion rate and chemical and thermal stability. The most important gas requirements in airbags are:
1. Proper volume and pressure: To create an effective airbag, the volume of gas and pressure inside the bag must be precisely calculated. Too much pressure can cause injury to the occupant, and too little pressure will not provide adequate protection.
2. Fast inflation speed: The gas must fill the bag in a short time to prevent the occupant from moving.
3. Thermal stability: Rapid expansion of a gas generates heat; the gas must be stable against this temperature increase.
4. Chemical stability: The gas should not react with the bag fabric, wires or metal parts and should not cause long-term damage to the system. Given these requirements, a combination of nitrogen and argon is an ideal choice. Nitrogen is light and fast, while argon is heavier and more stable, allowing for pressure and temperature control.
Physical and chemical properties of argon and nitrogen
Argon (Ar)
Argon is a noble gas whose properties make it suitable for use in airbags:
• Atomic mass and density: 39.95 and 1.784 g/L. This high molar mass slows the rate of gas diffusion and controls the pressure of the bag.
• Chemical properties: Completely neutral and non-flammable, no reaction with bag materials or metal components.
• Advantages:
o Control of internal bag pressure
o Reduce heat generation during expansion
o Long-term stability in storage conditions
o Safe and environmentally friendly
Nitrogen (N₂)
Nitrogen is a stable, non-flammable gas and is used in airbags due to the following properties:
• Molar mass and density: 28.02 and 1.2506 g/l. Nitrogen’s lightness allows the bag to fill quickly.
• Chemical properties: Very stable and non-reactive, does not react with the bag and metal components.
• Advantages:
o High expansion rate
o Affordable cost and availability
o Long stability in compressed or liquid form
Advantages of combining argon and nitrogen
Combining these two gases allows for the preservation of both the advantages of nitrogen and argon:
1. Precise control of expansion rate
2. Reduction of heat generation and protection of the fabric and system
3. Chemical stability and long life of the bag
4. Ability to adjust the ratios for different vehicle needs
5. Safe and environmentally friendly
Argon and nitrogen combination in airbags
Common Ratios
• 10–30% Argon and 70–90% Nitrogen
• More Argon for Side and Long Curtain Bags
• Less Argon for Front Bags Needing Rapid Expansion
Impact on performance
• Overpressure control
• Reduction of peak temperature at the moment of opening
• Increase bag life and prevent corrosion
• Uniform gas distribution in all sections
Vehicle system compatibility
• High pressure (200–300 bar) and wide temperature range (-40 to +85°C)
• Precision valves and systems to deliver the correct gas ratio
• Precision engineering to adapt to bag type and occupant position
Comparison with other gases
Instant gas production
| Gas | Advantages | Disadvantages |
| Pure Nitrogen | Fast speed, cheap | High pressure, less control |
| Helium | Light, fast | Expensive, leaks quickly |
| CO₂ | Instant gas production | Corrosive, toxic |
| Sodium Azide | Immediate Production | Hazardous, Environmental |
Advantages of Argon-Nitrogen Combination:
• Control of opening speed
• Thermal and chemical stability
• Safe and environmentally friendly
• Ability to adjust ratios
Airbag manufacturing and filling process
Gas Source
To fill air bags with a mixture of argon and nitrogen, gas sources must be available of the highest quality and purity:

1. Liquid or compressed argon
Argon is usually stored in two states: liquid at low temperatures (around −186°C) or compressed gas in high-pressure cylinders.
o Liquid argon storage has the advantage of less space and a high volume of gas in cold conditions, but requires safety and cryogenic equipment.
o Compressed argon gas is suitable for automotive systems that require smaller volumes and constant pressure, and is easier to maintain.
2. Liquid or compressed nitrogen
o Nitrogen is often used as the main filling gas due to its low cost and easy availability.
o Storage is done in liquid or compressed form, depending on the module design.
o Liquid nitrogen can quickly convert to gas and provide the volume needed to expand the bag.
How to inflate an airbag
The process of filling an airbag with a mixture of argon and nitrogen involves the following detailed steps:
1. Place the bag module in the filling chamber
o The module is placed in an isolated and controlled enclosure to prevent dust and moisture from entering.
o The ambient temperature and internal pressure of the chamber must be constant to maintain the exact gas composition ratio.
2. Injection of precise combination of argon and nitrogen
o Using precise measurement systems, the ratio of gases is adjusted based on the bag design.
o The gas flow enters the bag at controlled pressure so that the fabric and internal parts are not damaged.
3. Pressure and volume control
o The internal pressure of the bag and the volume of gas introduced are carefully measured to prevent explosion or incomplete filling.
o Electronic equipment and pressure sensors are used in this stage.
4. Leakage test, opening speed and gas ratio
o After filling, the bags undergo final tests:
Leak test: Ensures no gas escapes over time
Opening speed test: Checks bag performance under simulated crash conditions
Gas ratio test: Confirms that the argon and nitrogen composition remains exactly as specified
Safety measures
• Adhere to cryogenic protocols for liquefied gases, including the use of gloves, face shields, and protective clothing
• Use of valves and regulators resistant to pressure and temperature
• Prevent contamination and moisture ingress, as water and particles can cause chemical reactions or damage to the bag.
• Store bags in a cool, dry environment to maintain optimal gas performance.
Application in the automotive industry
The combination of argon and nitrogen in airbags is used in various types of bags:
• Front Airbag: Designed for the driver and front passenger
o Requires very fast and uniform filling
o The combination of argon and nitrogen allows for peak pressure and temperature control
• Side Airbag:
o Smaller volume, but requires higher precision
o Accurate throttle ratio control is critical to prevent side-impact injury to the occupant.
• Curtain Airbag: Long and wide to protect the head and neck
o Need for uniform gas distribution throughout the bag
o Argon in these bags reduces peak temperatures and controls pressure throughout the bag.
Industry Adoption
• Prestigious European and Japanese brands are using this technology
• Main motivation: meeting stringent safety standards and reducing environmental impact
• Increasing regulatory standards for occupant protection have increased the demand for argon-nitrogen airbags
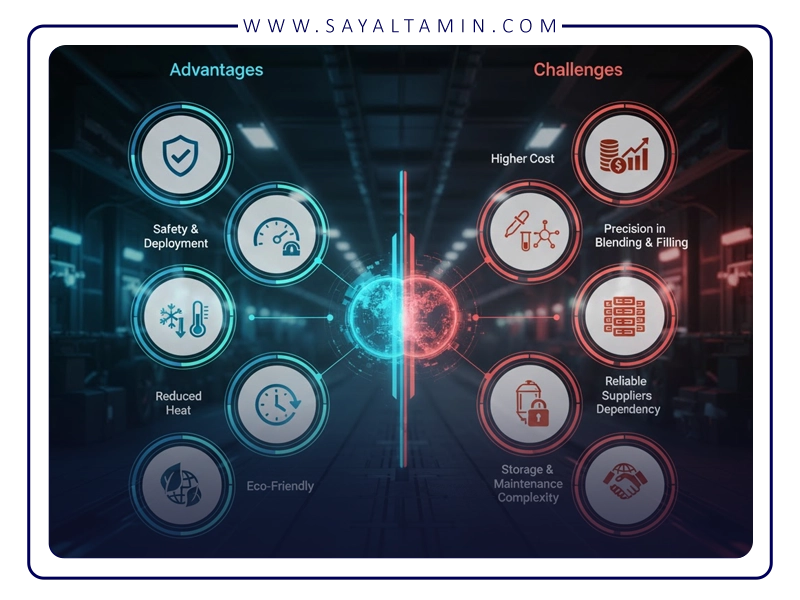
Benefits and challenges
Advantages
1. Safety and stability
o The composition is neutral and non-flammable, and the bag’s performance remains stable over time
2. Bag opening control
o Controlled expansion speed and pressure prevent injury to the occupant
3. Reduced heat generation
o Prevents damage to the fabric and internal components
4. Long life
o Stable composition ensures the bag’s performance even after years
5. Environmentally friendly
o Does not produce any toxic or polluting substances
Challenges
1. Higher cost than pure nitrogen
o Argon is relatively more expensive and liquid storage requires special equipment
2. Need for precision in mixing and filling
o The slightest error in gas ratio can disrupt bag performance
3. Complexity of storage and handling
o High pressure and low temperature require precise equipment and protocols
4. Dependence on reliable suppliers
o Gas quality and delivery time affect bag performance and occupant safety
Future trend
• Optimization of the mixture ratio: Research is underway to optimize the ratio of argon and nitrogen for different types of bags
• Combination with intelligent sensor systems: Sensors can dynamically adjust the gas ratio and bag opening time
• Combination of gas and chemicals: For ultra-fast bag opening in severe accidents
• Expansion of the gas condensate supplier network: Increasing the production and supply of argon and nitrogen to meet the global demand of the automotive industry
Argon and nitrogen are an advanced, safe and sustainable solution for airbags. This combination:
• Combines the rapid expansion rate of nitrogen with the pressure and temperature control of argon
• Optimizes bag performance and extends its life
• Reduces the risks of chemical reactions and high temperatures
For vehicle manufacturers, using this combination means improving occupant safety and meeting international standards. For gas suppliers, delivering a high-quality, pure product is key to commercial and industrial success.
————————————————–
References
1. SAE International, Airbag Systems and Gas Generation, SAE Technical Paper Series, 2020.
2. ISO 12097:2017, Road vehicles — Safety systems — Airbag modules.
3. ASTM D2501, Standard Specification for Compressed Inert Gases.
4. Smith, D., Automotive Safety and Airbag Design, Elsevier, 2019.
5. Brown, J., et al., “Inert Gas Mixtures in Airbag Inflation,” Journal of Automotive Safety, vol. 15, no. 3, 2021.
6. Johnson, R., & Lee, H., Advanced Airbag Technologies and Inert Gas Applications, Springer, 2022.
7. Miller, T., “Cryogenic Gas Handling for Automotive Safety Systems,” International Journal of Vehicle Safety, vol. 8, 2020.
8. European Commission, Regulations on Automotive Airbag Systems, 202