This post is also available in: Persian Armenian
Lyophilization or freeze-drying is a complex and crucial process for preserving the quality and longevity of heat-sensitive materials, including biological substances, pharmaceuticals, and food products. This method removes water through sublimation (the direct transition of ice into water vapor) under low temperatures and pressures, preventing heat-induced damage and preserving the physical, chemical, and biological properties of the product. In this process, cooling, and especially the use of liquid nitrogen, plays a vital and indispensable role. This article details the principles of lyophilization, the role of liquid nitrogen at each stage, the advantages, and the challenges of using it.
Principles of Lyophilization
The lyophilization process generally consists of three main stages: freezing, primary drying (sublimation), and secondary drying (adsorption). Each of these stages requires precise control of parameters such as temperature, pressure, and time.
- Freezing
In the freezing phase, the product is completely frozen. The main objective at this stage is to convert the water in the product into ice crystals. The quality of the ice crystals formed directly impacts the quality of the final product. The freezing rate is a key factor in this stage. Rapid freezing, using liquid nitrogen, prevents the formation of large ice crystals. Large ice crystals can damage the product structure, such as cell membranes, proteins, and complex molecular structures.
The benefits of rapid freezing include the formation of small and uniform ice crystals, which reduce cell and molecular damage. This method also helps to preserve the biological activity of sensitive materials like enzymes, antibodies, and vaccines. Furthermore, rapid freezing aids in maintaining the solubility and stability of the product in the subsequent drying stages.
- Primary Drying (Sublimation)
After complete freezing, the pressure inside the lyophilizer chamber is significantly reduced (usually to high vacuum, typically below 0.1 mbar) while simultaneously applying a small amount of heat to the product. Under these conditions, the ice in the product directly converts into water vapor through the process of sublimation. Precise temperature control at this stage is critical to avoid exceeding the eutectic point or collapse point and to prevent the collapse of the porous structure of the product.
One of the challenges in this phase is the partial melting of ice, which leads to collapse or the breakdown of the structure. If the ice partially melts, water vapor will not be able to effectively escape from the melted layers, reducing the efficiency of the process.
- Secondary Drying (Desorption)
In this phase, the remaining moisture, which is absorbed as bound water to the surfaces of the product, is removed. This moisture can be evaporated at temperatures lower than 100°C. More heat is applied to the product, and the pressure is maintained at high vacuum levels. The goal of this process is to reduce water activity and prevent chemical reactions and biological degradation.
By removing the remaining moisture, the long-term stability of the product is ensured, and unwanted reactions are prevented.
Role of Liquid Nitrogen in Lyophilization
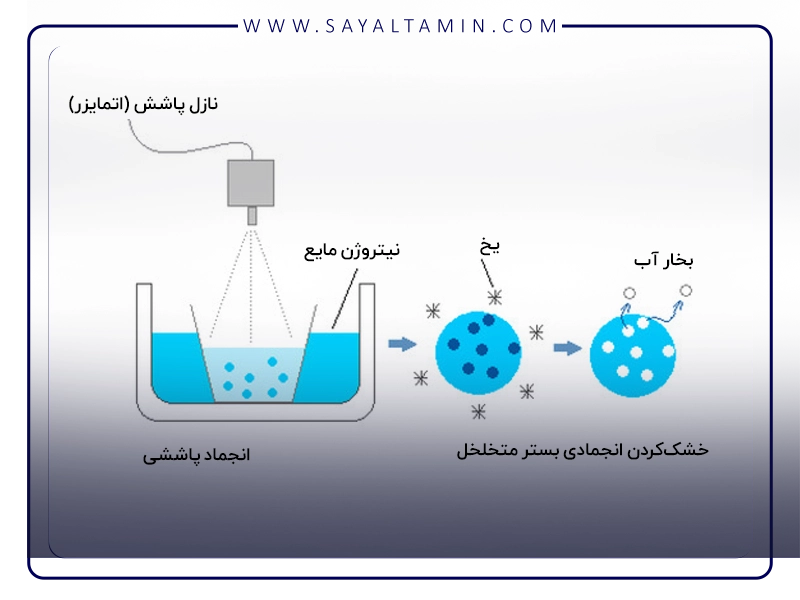
Liquid nitrogen (LN₂), with its extremely low boiling point (-196°C or 77 K), serves as an ideal cooling agent in each stage of the lyophilization process. Its unique properties, including ultra-low temperature, high cooling capacity, and chemical inertness, make it particularly suited for lyophilization.
- Rapid and Uniform Freezing (Flash Freezing)
Liquid nitrogen can be applied directly to the product (if suitable) or indirectly through cold surfaces (such as metal plates coated with LN₂) for fast cooling. The high thermal conductivity of LN₂ enables quick and efficient freezing of the sample.
The benefits of rapid freezing with liquid nitrogen include:
- Formation of small and amorphous ice crystals: Rapid freezing limits the time for water molecule migration and growth, resulting in the formation of very fine or amorphous ice crystals. This minimizes damage to the internal structure of the product.
- Preservation of biological activity: For biological materials like enzymes, antibodies, and living cells, maintaining the 3D structure and biological activity is crucial. Rapid freezing with LN₂ minimizes protein denaturation and damage to cell membranes.
- Improved sublimation efficiency: A well-frozen product that retains its porous structure releases water vapor more efficiently during sublimation, reducing the overall drying time.
- Reduced chemical reactions: Rapid freezing slows down chemical reactions in the materials and prevents product degradation.
- Maintaining Low Temperature in the Condenser (Cold Trap)
During the primary drying stage, water vapor sublimated from the product must be quickly removed from the chamber and converted into a solid (ice) to prevent it from re-entering the product or increasing pressure in the system. This is achieved using a cold trap. Advanced lyophilization systems use circulating liquid nitrogen in condenser coils to maintain very low temperatures (typically below -70°C and even as low as -100°C).
Benefits of using liquid nitrogen in the condenser include:
- High efficiency in water vapor absorption: The lower the temperature of the condenser, the lower the vapor pressure of water, allowing it to exit the chamber quickly and effectively freeze in the condenser.
- Prevention of vapor backflow: The extremely low temperature of the condenser prevents water vapor from returning to the chamber and contaminating the product.
- Maintenance of stable vacuum: Effective absorption of water vapor helps maintain a low and stable vacuum pressure in the chamber.
- Precise Temperature Control During the Process
During lyophilization, precise temperature control at different stages is essential. Liquid nitrogen, as a cooling or heating fluid, provides the ability to maintain the exact temperature at each stage of the process. This precise temperature control prevents partial melting (collapse) of the product during sublimation and optimizes the secondary drying phase.
| Read more: Use of liquid nitrogen in the construction and civil engineering industries |
Advantages of Using Liquid Nitrogen in Lyophilization
The use of liquid nitrogen in the lyophilization process offers numerous and significant advantages, making it a preferred option in many industries:
- Maximized product quality retention: Rapid freezing with liquid nitrogen helps preserve the cellular and molecular structure of the product, which is especially important for sensitive pharmaceutical and biological products.
- Increased efficiency and speed of the process: Rapid freezing and efficient condensers enhance the sublimation rate and reduce the overall drying time.
- Precise temperature control: Liquid nitrogen allows for exact temperature management, preventing product collapse.
- Long-term product stability: Lyophilization with liquid nitrogen ensures the quality and long-term stability of the final product.
Challenges and Considerations in Using Liquid Nitrogen
Despite its numerous advantages, using liquid nitrogen in lyophilization comes with certain challenges:
- High cost: The cost of liquid nitrogen consumption and the infrastructure required for its storage and transportation can be relatively high.
- Safety concerns: While liquid nitrogen is chemically inert, direct contact can cause severe frostbite. Additionally, adequate ventilation is essential in enclosed spaces.
- Infrastructure requirements: Storing and handling liquid nitrogen requires specialized tanks and complex piping systems, which can be costly for industrial-scale and large-scale processes.
| Read more: Nitrogen preserves and stores stem cells |
Liquid nitrogen plays a vital role as a powerful cooling agent in the lyophilization process. Its rapid cooling capability preserves product structure, biological activity, and overall quality. By using liquid nitrogen, the drying process is optimized, and products with long-term stability and shelf life are produced. Despite challenges related to cost and safety, the use of liquid nitrogen will continue due to its numerous benefits across various industries.
————————————————–
References




