This post is also available in: Persian Armenian
Oxygen is a chemical element with the atomic number 8 (it has eight protons in its nucleus), and it forms a molecular (O₂) structure composed of two oxygen atoms. At standard temperatures and pressures, it is a colorless, odorless gas.
Oxygen atoms are highly reactive and participate in a wide variety of common chemical compounds, readily combining with other elements.
About 21% of the Earth’s atmosphere consists of oxygen. However, this was not always the case, as Earth’s early atmosphere lacked oxygen. The oxygen present in today’s atmosphere was produced by microorganisms that synthesized their food through photosynthesis, releasing oxygen as a byproduct. This oxygen drastically altered the environment of our planet and the history of life.
Oxygen and Life
Oxygen is vital for life. Most living organisms use oxygen for cellular respiration, a process that enables cells to obtain energy. Plants also use oxygen for cellular respiration, but in a separate process called photosynthesis, they absorb carbon dioxide and release oxygen. The rate of photosynthesis is higher than respiration when there is ample light, resulting in a net production of oxygen, which is then released into the atmosphere.
Oxygen also plays a key role in the formation of ozone (O₃) in the Earth’s stratosphere, which helps block harmful ultraviolet (UV) radiation from the Sun. However, ozone in the troposphere is a dangerous pollutant.
Oxygen Gas Properties
- Oxygen exists as a diatomic molecule (O₂), meaning it consists of two oxygen atoms chemically bonded together.
- Colorless, odorless, non-toxic, and inflammable under normal conditions.
Uses of Oxygen
When oxygen is liquefied, it becomes easier to store and transport. However, the majority of applications use oxygen in its gaseous form.
Some of the primary uses of oxygen are:
- Healthcare and Medicine: Oxygen is critical in medical treatments, particularly for respiratory support in hospitals.
- Rocket Propulsion: Liquid oxygen is used as a propellant in rocket engines.
- Metal Industry: Oxygen is used in combination with acetylene and other fuel gases for metal cutting, welding, hardening, cleaning, and melting.
- Steel and Iron Manufacturing: Oxygen or oxygen-enriched air is widely used to influence refining processes, removing carbon and facilitating oxidation reactions.
- Chemical and Petrochemical Industries: Oxygen is used as a feedstock in reactions with hydrocarbon building blocks to produce chemicals like alcohols and aldehydes.
- Pulp and Paper Industry: Oxygen is employed as a bleaching and oxidizing agent.
- Glass, Aluminum, Copper, Gold, Lead, Cement Manufacturing: Oxygen is used to enhance the combustion process, increasing efficiency and temperature in these industries.
- Waste Management: Oxygen is utilized to improve the efficiency of waste treatment plants.
- Aquaculture: Fish farms benefit from oxygen-enriched environments, which support better health and growth of the aquatic organisms.
- Diving and Mountaineering: Oxygen is essential in deep-sea diving and high-altitude mountaineering to support breathing.
 |  |  |
Health Effects of High Oxygen Concentration
The air around us contains about 21% oxygen, and oxygen is generally non-toxic. However, exposure to higher concentrations of oxygen can lead to certain health issues:
- Breathing pure oxygen at 80% at 1 atmosphere for more than 12 hours can cause symptoms such as respiratory irritation, reduced vital capacity, coughing, nasal congestion, sore throat, chest pain, and eventually pulmonary edema (fluid accumulation in the lungs).
- Breathing pure oxygen at pressures greater than 2 or 3 atmospheres can cause a neurological syndrome with symptoms like nausea, dizziness, vomiting, fatigue, lightheadedness, mood changes, euphoria, confusion, coordination issues, muscle contractions, burning sensations, pins and needles (especially in the fingers and toes), and loss of consciousness. In severe cases, seizures similar to epilepsy may occur, potentially leading to death.
- Premature infants receiving oxygen in incubators at concentrations higher than atmospheric levels may suffer irreversible eye damage. After about six hours of exposure to elevated oxygen levels, the retinal blood vessels may constrict, and if not promptly corrected, permanent damage can occur.
Oxygen Storage and Transport
Oxygen is stored in high-pressure cylinders or tanks, designed according to specific codes and standards regarding pressure and temperature. The volume of gas a cylinder can hold is determined by both the pressure and internal volume of the cylinder.
Storage Requirements:
- Oxygen cylinders must always be stored vertically in a well-ventilated, cool, dry, and secure location, ideally fire-resistant.
- No part of the cylinder should be exposed to temperatures exceeding 125°F (52°C).
- Cylinders should be kept away from high-traffic areas and emergency exits.
- Avoid storage near corrosive materials such as salt.
- When emptying cylinders, ensure the valve is closed and a slight positive pressure remains. Label the cylinders as “Empty” and never store full and empty cylinders together.
- Install “No Smoking” and “No Open Flames” signs where cylinders are stored or used.
- Cylinders should be separated from flammable materials according to safety standards and regulations.
Handling of Cylinders:
- Never drop, roll, or slide cylinders. Use a specialized trolley for transportation.
- Always handle cylinders with care to avoid damaging the valve or fittings.
- Never expose cylinders to oil, grease, or other combustible materials.
- Use oxygen-compatible lubricants only when necessary.
- Always open cylinder valves slowly to avoid rapid pressure release and minimize the risk of accidents.

- Cylinders should always be stored vertically and in a well-ventilated, dry, cool, secure and preferably fireproof location.
- No part of the cylinder may be heated above 125°F (52°C), and the storage area for oxygen cylinders must be free of combustible materials. Never overheat a cylinder to increase its pressure or increase the rate of discharge.
- Cylinders should be stored away from high traffic areas and emergency exits.
- Avoid storing in areas where salt and other corrosive substances are present.
- When emptying cylinders and reducing the gas pressure inside, make sure the valve is closed and some positive pressure remains in the cylinder. Also, label the cylinder as “empty” and never store full and empty containers together.
- Install “No Smoking” and “No Open Flame” signs in areas where cylinders are stored and used.
- Oxygen cylinders must be separated from flammable and combustible materials by distances specified by standards and regulations.
- Never drop, pull, or roll cylinders. Always use a specially designed hand cart or trolley to move the cylinder.
- Never lift a cylinder with its cap and lid on.
- Never allow substances such as oil, grease, or easily flammable materials to come into contact with the cylinders or valves.
- You are only allowed to use oxygen-compatible lubricants.
- Always open the compressed gas cylinder valve slowly to prevent rapid release of system pressure and accidents.
- Never use objects such as wrenches, screwdrivers, drills, etc. to loosen the cylinder head or cap. Doing so may cause damage. Only use a strap wrench specifically designed to loosen overly tight or rusty caps.
- Never tamper with safety devices on valves or cylinders.
- Always use a separate pressure reducing regulator or control valve along with properly designed pressure relief devices to safely vent gas to working systems.
- Adequate ventilation must be provided where oxygen is used. If the concentration of oxygen in the atmosphere increases, materials burning in air can burn more vigorously or even explosively, resulting in increased potential exposure to personnel and materials.
- It is important to note that the chemistry of fire begins to change in oxygen-rich environments. The International Organization for Standardization (ISO) defines an oxygen-rich atmosphere as any atmosphere containing more than 23.5 percent oxygen. As a result, materials that ignite readily in air are not only more susceptible to ignition but also burn more intensely in enriched environments. This includes clothing and hair, which have porous spaces to trap oxygen.
- Personnel must be fully familiar with the properties and safety precautions before being allowed to work with oxygen and its related equipment.
- When working with cylinders, wear safety glasses, safety shoes, and leather work gloves.
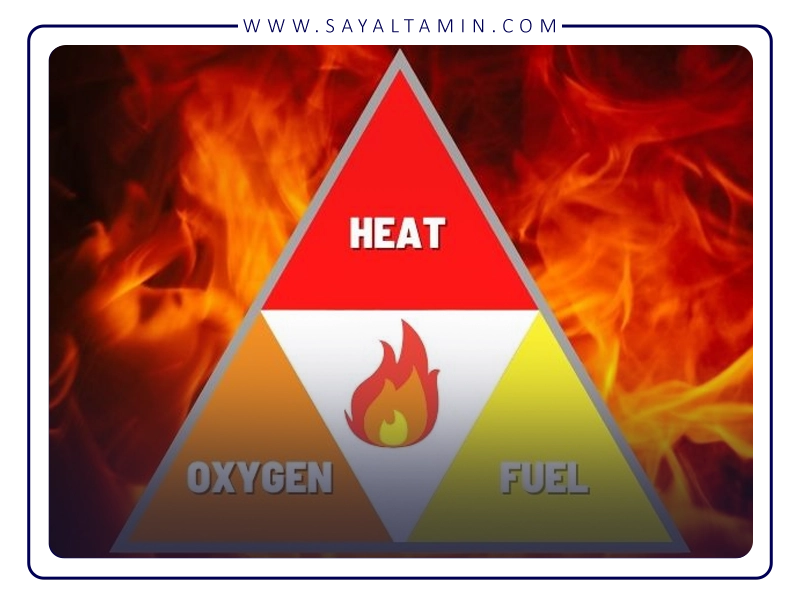
Fire and Oxygen
Oxygen itself is non-combustible and does not burn, but it is essential for combustion. Oxygen is one of the three elements of the fire triangle, alongside fuel and heat. The heat produced by burning fuel and oxygen sustains the fire, continuing until both fuel and oxygen are available.
Combustion Reaction:
- The chemical reaction of burning can be described as:
Substance being burned + Oxygen —–> Products + Heat
- For example, burning carbon in oxygen produces carbon dioxide and releases heat:
Element + Oxygen —–> Oxide of Element + Heat
C(s) + O2(g) —–> CO2(g) + Heat
Carbon + Oxygen —–> Carbon Dioxide + Heat
2Hg (I) + o2 (g) —–> 2Hgo (s) + Heat
Mercury + Oxygen —–> Mercury (II) Oxide + Heat