This post is also available in: Persian Armenian
Diamond, one of the most unique naturally occurring materials, has always held a prominent place in advanced technologies, tooling, electronics, optics, medicine, and even energy sectors. Historically regarded solely as a precious gemstone, advances in science and engineering over recent decades have made synthetic diamond not only a substitute for natural diamond but, in many applications, a superior performer.
Among various methods for synthetic diamond production, Chemical Vapor Deposition (CVD) is recognized as the most precise, controllable, and advanced technique. CVD allows the production of diamond layers and crystals with high purity, engineered crystalline structures, and tailored physical–chemical properties.
In this process, the role of industrial gases—especially inert gases—is decisive. Among them, argon (Ar), a noble gas, appears chemically inert but plays a key role in plasma stability, energy control, growth uniformity, and final diamond quality.
Understanding Synthetic Diamond and Its Difference from Natural Diamond
Natural diamonds form over millions of years deep within the Earth under extreme pressure and temperature. While this natural process produces a highly hard and stable product, it inherently faces limitations such as heterogeneity, impurities, and limited control over material properties.
In contrast, synthetic diamonds recreate these thermodynamic growth conditions in controlled laboratory or industrial environments. The primary advantage of this approach is the ability to engineer diamond properties based on the intended application, from optical transparency to thermal conductivity, wear resistance, and even electronic characteristics.
Synthetic diamonds are typically produced by two main methods:
- High Pressure–High Temperature (HPHT)
- Chemical Vapor Deposition (CVD)
While HPHT simulates natural geological conditions, CVD relies on plasma chemistry and gas-phase reactions, and due to its precision, it is preferred for many advanced applications today.

Overview of the CVD Process for Diamond Synthesis
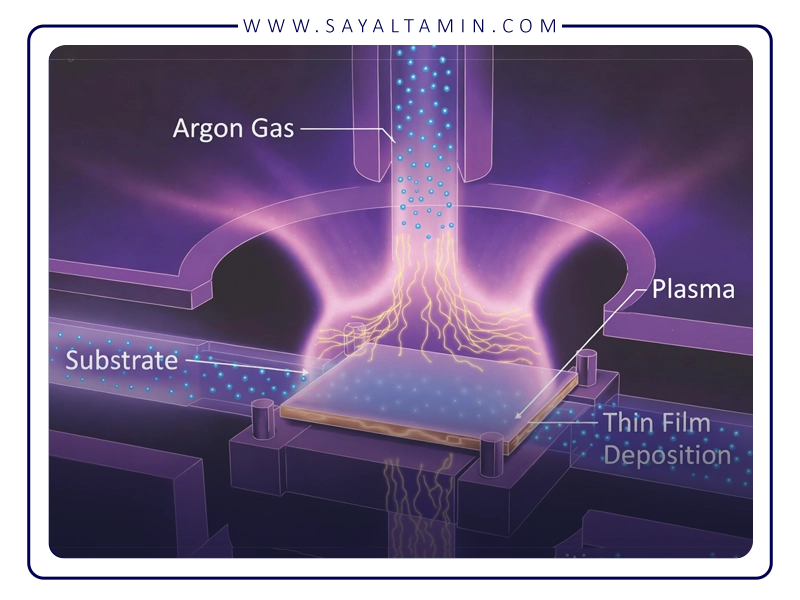
In CVD, diamond growth occurs via decomposition of carbon-containing gases in an active environment, usually a plasma or high thermal energy field, which breaks molecular bonds and releases reactive carbon atoms.
The primary stages of the CVD diamond process are:
- Injection of a gas mixture (typically methane and hydrogen)
- Activation of gases using plasma or heat
- Deposition of carbon atoms onto a substrate
- Gradual growth of the diamond crystal structure
Precise control of gas composition, temperature, pressure, and plasma stability is critical for diamond quality. Any fluctuations can lead to unwanted phases such as graphite or amorphous carbon.
Here, the role of inert gases like argon becomes crucial.
Argon: A Noble Gas with Roles Beyond Inertness
Argon is a noble gas constituting roughly 0.93% of Earth’s atmosphere. Its stable electronic structure makes it highly unreactive, which is why it is widely used as a protective environment, plasma stabilizer, and carrier gas in various industries.
At first glance, it may seem counterintuitive to use an inert gas in a process driven by active chemical reactions. The answer lies in argon’s indirect yet critical contributions.
In CVD diamond synthesis, argon:
- Enhances plasma stability and uniformity
- Distributes reaction energy in a controlled manner
- Regulates diamond crystal growth rate and morphology
- Prevents undesired side reactions
Gas Mixture in CVD and Argon’s Position
| Gas | Primary Role | Typical Volume Fraction |
| Hydrogen (H₂) | Remove non-diamond phases, stabilize diamond structure | 85–99% |
| Methane (CH₄) | Carbon source for diamond growth | 0.1–5% |
| Argon (Ar) | Plasma stabilization and energy control | 0–50% (process-dependent) |
Contrary to common assumptions, higher argon fractions in some CVD systems can improve surface quality, growth uniformity, and reduce internal stress in the diamond layer.

Argon’s Role in Plasma Stability
Plasma, an ionized gas containing electrons, ions, and reactive radicals, directly determines diamond growth quality. Argon, due to its suitable ionization energy and relatively high atomic mass, is ideal for supporting plasma.
Its presence ensures:
- More stable and uniform plasma
- Reduced energy fluctuations
- Homogeneous temperature distribution in the reaction chamber
This is especially critical in Microwave Plasma CVD (MPCVD), where precise electromagnetic and plasma control is required.
Argon’s Influence on Diamond Morphology and Crystal Structure
Controlling surface morphology and crystal orientation is a major challenge in CVD diamond production. Argon can indirectly affect these properties:
- Slows excessive growth, leading to smoother surfaces
- Promotes fine and uniform grain formation
- Reduces the probability of crystal defects
This is particularly important for diamond thin films used in electronics and optics.
Temperature Control and Thermal Stress Management
Diamond growth occurs at high temperatures (700–1100°C), and minor thermal fluctuations can induce internal stress, microcracks, or phase conversion to graphite.
Argon, with its higher heat capacity and atomic mass, helps distribute thermal energy evenly in the chamber. Acting as a “thermal buffer,” it absorbs excess plasma energy and prevents localized overheating.
For thick or large diamond substrates, this reduces cumulative stress, preventing layer delamination or cracking.
Argon in Chemical Purity and Minimizing Unwanted Carbon Phases
Achieving pure sp³ diamond phase and avoiding sp² graphite or amorphous carbon is crucial. While hydrogen’s role is well-known, argon indirectly contributes by:
- Reducing the density of highly energetic radicals
- Controlling surface reactions
- Limiting unwanted sp² bond formation
Higher argon ratios in advanced systems can even improve optical transparency, as fewer graphite impurities reduce light absorption.

Comparative Analysis: With vs. Without Argon
| Process Parameter | Without Argon | With Argon |
| Plasma stability | Moderate, prone to fluctuation | High, uniform |
| Temperature control | Difficult | Precise, stable |
| Surface quality | Rougher | Smoother |
| Graphite formation | Higher | Lower |
| Internal stress | Higher | Reduced |
| Growth uniformity | Limited | Improved |
Argon functions as an environmental optimizer, enhancing overall product quality.
Argon in Various CVD Systems
- MPCVD: Stabilizes electromagnetic field, allows plasma at lower pressures → better process control, lower energy consumption
- HFCVD: Reduces filament corrosion, extends equipment lifetime, minimizes unwanted deposits
- Plasma Jet/Hybrid CVD: Shapes plasma jet, controls growth area, enables complex diamond structures

Industrial Applications Dependent on Argon
CVD diamond quality directly determines industrial applicability:
- Cutting and wear tools: High thermal stability and low stress → longer life, uniform performance
- Electronics and semiconductors: High purity, low defect diamonds for heat sinks and future semiconductors
- Advanced optics and lasers: Transparency and uniformity improved by argon, reducing light absorption by graphite
Argon in Industrial Gas Supply Chains
Stable, high-purity argon supply is essential, with minimal oxygen, nitrogen, or moisture impurities. Industrial gas providers, particularly in energy and gas condensates, play a strategic role.
Argon is not merely a supplemental gas but an integral component of advanced CVD diamond infrastructure.

Economic Considerations
While adding argon may appear as an extra cost, industrial experience shows that smart argon use reduces final production costs by:
- Reducing defect rates → lower waste, optimal use of substrates, energy, and time
- Increasing equipment lifetime → reduced maintenance costs
- Providing operational flexibility → maintaining quality at different pressures and plasma powers
Safety and Operational Considerations
Argon is non-toxic and non-flammable but is a simple asphyxiant, so adequate ventilation, oxygen sensors, and personnel training are essential.
High-purity argon is critical: moisture or impurities can negatively affect plasma stability and diamond growth.
Emerging Trends
- Higher argon ratios for nanostructured or ultra-thin diamond films → precise control of surface energy and morphology
- Argon combined with other noble gases (Ne, He) → tailored plasma parameters for advanced diamond properties
- Growing demand in quantum electronics, advanced sensors, and high-power thermal management → increasing high-purity argon consumption
CVD diamond synthesis exemplifies the synergy between materials science, plasma chemistry, and industrial gas engineering.
Although chemically inert, argon is crucial for optimizing processes, enhancing product quality, and improving operational stability.
Its effects include:
- Plasma stability
- Temperature control
- Reduced internal stress
- Improved surface morphology
- Minimization of unwanted carbon phases
Industrially, argon use is a strategic and economic decision, reducing waste, extending equipment life, and providing operational flexibility.
Understanding argon’s role allows industrial gas suppliers to offer precise solutions for advanced industries, strengthening their position in the value chain of next-generation technologies.
————————————————–
References
1. Butler, J. E., & Sumant, A. V. (2008). The CVD of diamond for electronic devices. Chemical Vapor Deposition, 14(7–8), 145–160.
2. May, P. W. (2000). Diamond thin films: a 21st-century material. Philosophical Transactions of the Royal Society A, 358(1766), 473–495.
3. Tallaire, A., Achard, J., & Silva, F. (2014). Chemical vapor deposition diamond growth. Comptes Rendus Physique, 15(2), 169–184.


