This post is also available in: Persian Armenian
The air around us is a mixture of several main gases, the most important of which are nitrogen (about 78%), oxygen (about 21%) and argon (about 0.9%). Although these gases are naturally mixed in the atmosphere, many industries require them in separated and purified form.
On an industrial scale, separation of these gases is achieved by distilling liquid air at very low temperatures. This method, carried out in units known as Air Separation Units (ASU), is considered one of the most advanced cryogenic processes in the world. This article explains, in a comprehensive yet practical way, how oxygen, nitrogen and argon are separated from ambient air and then converted into their liquid form. At the end, the main applications and economic importance of each gas are also highlighted.
Composition and main properties of the gases in air
Before looking at the separation process, it is useful to review the composition of air:
| Gas | Volume % in air | Boiling point (at 1 atm) | Main applications |
| Nitrogen (N₂) | 78.08% | −195.8 °C | Refrigerant, metal blanketing, ammonia production |
| Oxygen (O₂) | 20.95% | −183 °C | Combustion support, medical use, steelmaking |
| Argon (Ar) | 0.93% | −185.8 °C | Shielding gas in welding and laser applications |
| Other gases (CO₂, Ne, He, …) | < 0.05% | Various | Special laboratory and niche industrial applications |
As seen above, the differences in boiling point between these gases form the basis of fractional distillation in air separation.
Main stages of the air separation process
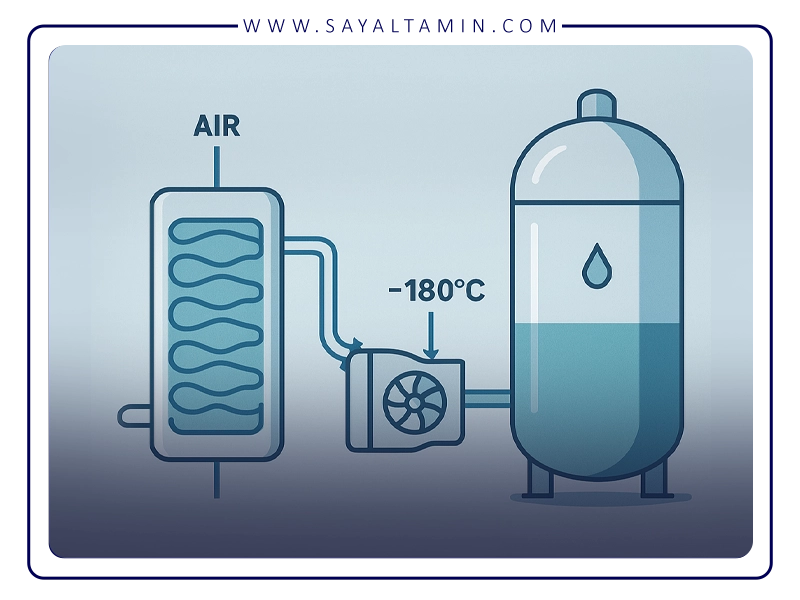
1. Filtration and compression of air
In the first step, ambient air is drawn into the separation unit. This air normally contains dust, moisture and other impurities. To protect downstream equipment, it is first passed through mechanical filters and moisture traps to remove solid particles and most of the water.
The cleaned air then enters powerful compressors and is compressed to a pressure of about 6–10 bar. Compression is the first step in preparing the air for subsequent cooling and liquefaction.

2. Removal of moisture and carbon dioxide
In the second stage, moisture and carbon dioxide must be removed from the air, because at cryogenic temperatures they would freeze and block lines, valves and column internals.
This purification is carried out in adsorption towers filled with materials such as molecular sieve (zeolite) or activated alumina. Water vapour and CO₂ molecules are captured on the surface of the adsorbent, and a dry and purified air stream leaves the outlet.
Typically, twin-tower (swing) systems are used so that one bed operates while the other is regenerated.
3. Cooling and liquefaction of air
In the next step, the dried air must be cooled down to very low temperatures (below −180 °C). For this purpose, heat exchangers and expanders (turbo-expanders) are used.
Part of the compression energy stored in the air is later recovered in the expander. As the air expands and does work, its temperature drops sharply. In combination with regenerative heat exchange against cold product streams, this cooling is sufficient to partially or fully liquefy the air.
The resulting liquid air, which is still a mixture of oxygen, nitrogen and argon, is then fed to the distillation section.

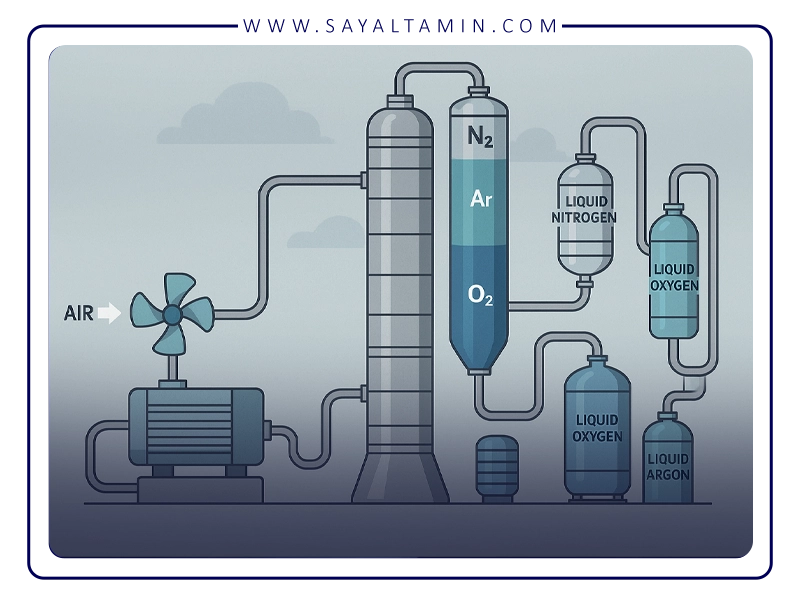
4. Distillation of liquid air – the heart of the process
Distillation of liquid air is the core and most sensitive part of the entire separation process. It is carried out at very low temperatures and controlled pressures, with the aim of separating the components of air based on their different boiling points.
As noted earlier, nitrogen boils at −195.8 °C, oxygen at −183 °C and argon at about −185.8 °C. These relatively small differences in boiling point are enough to enable fractional distillation when many stages of vapour–liquid contact are provided.
In industrial ASUs, the process is typically carried out in two main columns:
1. High Pressure Column (HPC)
2. Low Pressure Column (LPC)
In many modern plants, a side argon column is also added to efficiently recover argon.
a) High Pressure Column (HPC)
Liquid air, cooled in the main heat exchanger, is introduced near the bottom of the high pressure column. The column is equipped with trays or structured/random packing to promote intimate contact and mass/heat transfer between rising vapour and descending liquid.
In the lower part of the column, the liquid is colder and richer in oxygen (the heavier component), while the lighter, nitrogen-rich vapour rises towards the top. As the vapour and liquid exchange heat and mass across each stage, the nitrogen concentration gradually increases in the upper sections and oxygen concentration increases in the lower sections.
In effect, each tray or packing segment establishes a local equilibrium, and by stacking many such stages, a high degree of separation is obtained.
At the top of the HPC, an overhead nitrogen-rich vapour stream is withdrawn. Depending on plant design, part of this stream can be used as high-pressure nitrogen product or sent to the low pressure column for further purification. The oxygen-rich liquid at the bottom of the HPC becomes the feed to the low pressure column.

b) Low Pressure Column (LPC)
The low pressure column operates typically at about 1.2–1.5 bar and is responsible for achieving the high purity levels required for the final oxygen and nitrogen products.
Cold liquid and vapour streams from the HPC are fed into the LPC. Again, vapour–liquid contact takes place over many stages:
. Light nitrogen accumulates at the top of the column.
. Heavy oxygen collects at the bottom as liquid.
The final overhead stream from the LPC is high-purity nitrogen, often with a purity above 99.99%, while the bottom stream is liquid oxygen with a purity typically higher than 99.5%.
The LPC is thermally coupled to the HPC through a condenser–reboiler located between the two columns. In this integrated exchanger, nitrogen vapour from the top of the HPC is condensed, giving up its latent heat. That heat is simultaneously used to boil a portion of the oxygen-rich liquid at the bottom of the LPC. This tight thermal coupling stabilises the continuous evaporation–condensation cycle in both columns and ensures energy efficiency.
c) Argon column
Because the boiling point of argon is very close to that of oxygen, its separation is more challenging and requires a dedicated distillation system.
In the middle section of the LPC, a side-draw vapour stream can be found that contains about 9–12% argon, along with oxygen and a small amount of nitrogen. This argon-enriched stream is fed to a side argon column.
In the argon column, the same principles of fractional distillation apply, but the design and number of stages are optimised to increase the argon concentration to above 99.99%.
To reach such high purities, polishing units are often used downstream. One common method is to react residual oxygen with a small amount of hydrogen over a catalyst to form water, which is then removed, leaving ultra-high-purity argon.
As a result, the air separation unit delivers three main products:
– High-purity nitrogen from the top of the LPC,
– High-purity oxygen from the bottom of the LPC,
– High-purity argon from the side argon column.

5. Storage and final liquefaction
After separation, the product gases are typically stored and supplied in two main forms:
1. Compressed gas in cylinders or high-pressure storage vessels.
2. Cryogenic liquid in insulated, low-temperature storage tanks.
Liquefaction is achieved using multi-stage refrigeration systems. The gas passes through several stages of cooling, expansion and heat exchange until it reaches its boiling point and condenses to a liquid.
Cryogenic storage tanks
Liquid gases are stored in special double-walled, vacuum-insulated tanks:
The space between the inner and outer shell is evacuated and often filled with insulating material such as perlite to minimise heat transfer.
Each gas is stored near its own boiling temperature:
Liquid nitrogen around −196 °C
Liquid oxygen around −183 °C
Liquid argon around −186 °C
For transport, cryogenic road tankers are used. They are similar in design to stationary tanks but are reinforced to withstand road vibrations and pressure fluctuations. At the point of use, the liquid gas is passed through ambient vaporizers or heated vaporizers, where it reverts to the gaseous phase and is then fed into process lines at the required pressure.

Safety systems in air distillation (ASU) plants
Air separation units operate under extreme temperature and pressure conditions, so process control and safety are absolutely critical.
1. Pressure and temperature control
– Relief valves are installed to prevent overpressure in lines and vessels.
– Automated control systems (PLC / DCS) continuously monitor temperature, pressure, level and flow at key points throughout the plant and intervene in case of deviation.
2. Contamination control and explosion prevention
Any contamination with oil or hydrocarbons in equipment handling oxygen can be extremely hazardous, as oxygen-enriched environments greatly increase the risk of ignition and violent combustion.
Therefore:
– All piping, compressors, valves and fittings in oxygen service must be of suitable, non-sparking materials (often stainless steel or aluminium).
– No organic grease or oil may be used in these systems; only oxygen-approved lubricants and cleaning procedures are allowed.

3. Protection against cold burns
Because of the very low temperatures of cryogenic liquids, direct contact with skin can cause severe frostbite and cold burns.
Operators must therefore use appropriate personal protective equipment (PPE):
– Insulated clothing,
– Cryogenic gloves,
– Face shields or goggles,
– Safety footwear resistant to low temperatures.
4. Safety in storage and transport
– Storage areas must be well ventilated. Leakage of inert gases such as nitrogen can displace oxygen in the air and create a risk of asphyxiation.
– Tanks are equipped with high/low level alarms, pressure monitoring and safety valves to prevent overfilling, excessive boil-off or overpressure.
Oxygen (O₂)
Oxygen is the most reactive major component of air and plays a vital role in more than 100 industries:
– Steel and metallurgy: To increase flame temperature and improve combustion efficiency in furnaces and converters.
– Medical and healthcare: In hospitals and clinics for respiratory therapy, anaesthesia and intensive care (ICU) systems.
– Chemical industry: In the production of ethylene oxide, methanol, acetaldehyde, nitric acid and other key intermediates.
– Environmental applications: To enhance biological activity and treatment efficiency in wastewater plants and aeration basins.

Nitrogen (N₂)
Nitrogen is one of the most inert and stable industrial gases and is widely used wherever a protective or cooling atmosphere is needed:
• Food industry: For nitrogen flushing and modified atmosphere packaging to prevent oxidation and spoilage.
• Petrochemical plants and refineries: For purging lines and vessels and preventing explosive atmospheres.
• Electronics and semiconductors: To prevent oxidation during soldering, chip fabrication and heat treatment of sensitive components.
• Cryogenic and research applications: As a powerful refrigerant for laboratory experiments, material testing and superconductivity research.
Argon (Ar)
Argon is a noble gas and chemically inert, which makes it ideal for applications requiring a completely neutral atmosphere:
• Welding of sensitive metals (TIG/MIG): Protects molten metal from contact with oxygen and nitrogen, preventing oxidation and nitrogen pickup.
• Glass and steel industries: Used as a protective blanket in critical stages to avoid surface oxidation.
• Electronics and laser technology: As a fill gas in argon lasers, discharge lamps and various plasma processes.
• Preservation of cultural heritage: Used to create oxygen-free atmospheres around historical artefacts and ancient documents to slow down degradation.
The cryogenic distillation of air is a remarkable achievement of chemical and mechanical engineering that enables precise separation of air components on an industrial scale.
By carefully controlling pressure, temperature and heat exchange, it is possible to extract three key industrial gases — oxygen, nitrogen and argon — from ordinary air in highly pure liquid or gaseous form.
These gases are not only fundamental to many modern industries such as steel, chemistry, energy and electronics, but also essential in medicine, environmental protection and advanced technologies that will shape the future.
————————————————–
References
1. Air Products and Chemicals, Inc. — “Cryogenic Air Separation Units: Principles and Operation”, Technical Bulletin, 2023.
2. Linde Engineering — “Air Separation Plants: Design, Operation, and Optimization”, Linde Technical Paper, 2022.
3. Praxair (Linde Group) — “Production of Oxygen, Nitrogen and Argon by Cryogenic Distillation”, Process Description Manual, 2021.
4. Air Liquide — “Industrial Gases: Oxygen, Nitrogen and Argon – Production and Applications”, Air Liquide Encyclopedia of Gases, 2023.
5. Smith, R. (2020). Chemical Process Design and Integration, 2nd Edition, Wiley.
6. Kister, H. Z. (2019). Distillation Design, McGraw-Hill Education.
7. Coulson & Richardson. (2022). Chemical Engineering, Volume 6: Design of Distillation Columns and Gas Separation, Elsevier.
8. Engineering Toolbox — “Physical Properties of Cryogenic Gases”, Online Reference, updated 2024.
9. Perry, R. H., & Green, D. W. (2021). Perry’s Chemical Engineers’ Handbook, 9th Edition, McGraw-Hill.
10. Ullmann’s Encyclopedia of Industrial Chemistry (2020) — “Air Separation and Industrial Gas Production”, Wiley-VCH.