This post is also available in: Persian Armenian
Volatile Organic Compounds (VOCs) are a diverse group of carbon-based chemicals characterized by high vapor pressure and low boiling points, which allows them to easily evaporate under environmental or process conditions. Their presence in industrial process streams and wastewater poses serious environmental, health, and operational concerns. Sectors such as petrochemicals, pharmaceuticals, electronics manufacturing, and coating industries generate significant amounts of VOCs—both as target products and as byproducts of various processes.
The separation and recovery of VOCs are essential not only for meeting environmental regulations but also for maintaining product quality and process safety. Among the available technologies, the use of nitrogen gas for stripping and purging operations offers distinct advantages—particularly in situations where oxidation must be prevented or the formation of explosive mixtures avoided.
Introduction
Volatile Organic Compounds (VOCs) are produced in nearly all branches of the chemical industry. Their volatility allows them to easily transfer into the gas phase, contributing to the formation of ground-level ozone, photochemical smog, and both indoor and outdoor air pollution. Many VOCs—such as benzene, methylene chloride, and chloroform—are toxic or carcinogenic, and prolonged exposure can have serious health effects on workers and surrounding communities.
From a process engineering perspective, the uncontrolled presence of VOCs can pose significant safety hazards—especially when their vapor concentrations fall within flammable limits. In addition, certain VOCs can degrade sensitive products through oxidation or contamination. This makes their control a dual priority: protecting human health and the environment while preserving product quality.
Nitrogen gas, due to its chemical inertness, low solubility in liquids, and ability to displace oxygen, plays a crucial role as a stripping medium in the removal of VOCs. This technology is particularly valuable in cases where the use of air could introduce safety risks, oxidation reactions, or undesirable changes in product quality.
Volatile Organic Compounds (VOCs) are a diverse group of carbon-based chemicals that readily evaporate under environmental or process conditions due to their high vapor pressure and low boiling points. The presence of these compounds in industrial streams and effluents poses not only environmental challenges but also serious health and operational concerns. Various industries—including petrochemical, pharmaceutical, electronic component manufacturing, and coating production—generate significant amounts of VOCs, either as target products or as process byproducts. The separation and recovery of these compounds are essential not only for complying with environmental regulations but also for maintaining product quality and ensuring process safety.
The use of nitrogen gas in stripping and purging operations offers distinct advantages due to its chemically inert nature, low solubility in liquids, and ability to displace oxygen—particularly in situations where oxidation must be prevented or explosive mixtures avoided. This paper examines the theoretical principles, engineering design considerations, and practical applications of nitrogen gas in the removal of VOCs from chemical processes and wastewater treatment systems, while also evaluating the environmental, safety, and economic implications of this technology.
Fundamentals of VOC Separation by Gas Stripping
Gas stripping is a common method used to transfer volatile compounds from the liquid phase to the gas phase, based on mass transfer between the two phases. This process relies on Henry’s law, which defines a direct relationship between the concentration of a substance in the liquid phase and its concentration in the gas phase at a given temperature. When a VOC-contaminated liquid comes into contact with nitrogen gas, the concentration gradient drives VOC molecules to move toward the gas phase, resulting in their increased concentration within the gas stream.
Several factors influence the efficiency of gas stripping. These include increasing the nitrogen flow rate, raising the process temperature to enhance the vapor pressure of VOCs, enlarging the gas–liquid contact area using specialized equipment such as packed towers or tray columns, and, in some cases, reducing system pressure to improve the volatility of high-boiling compounds. In process design, it is essential to maintain an appropriate balance between separation efficiency and operating costs.
Although ordinary air is often used for stripping due to its low cost, the presence of oxygen introduces several challenges. Oxygen can lead to the oxidation of sensitive compounds, the formation of unwanted byproducts, increased risk of explosions in the presence of flammable VOCs, and accelerated corrosion of metal equipment. Nitrogen gas provides an oxygen-free environment, mitigating these issues and enabling the safe removal of VOCs.
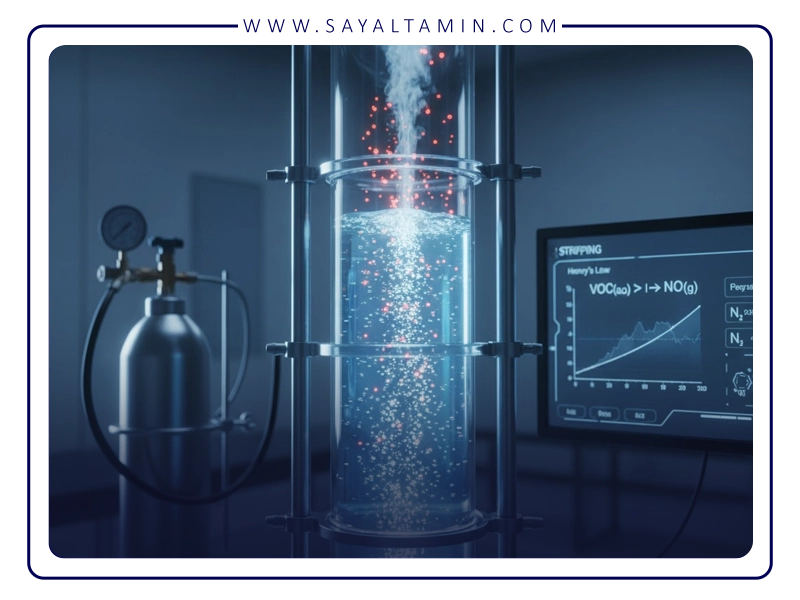
Applications of Nitrogen in Chemical Processes
In the chemical industry, particularly in the production of pharmaceuticals, dyes, and polymers, many intermediate or final products are highly sensitive to oxidation. Even small amounts of oxygen can lead to degradation, reduced purity, and altered product properties. For example, in the manufacture of bioactive pharmaceuticals, residual solvents such as dichloromethane are removed under a nitrogen atmosphere to prevent oxidation and preserve the quality of the active ingredient. In the polymer industry, the removal of unreacted monomers using nitrogen prevents polymer chain breakage and maintains the material’s mechanical properties.
One of the key advantages of nitrogen is the possibility of recovering separated VOCs. In many industries, compounds such as toluene, methyl ethyl ketone, or isopropanol can be recovered after nitrogen stripping using distillation or adsorption equipment and returned to the production cycle. This not only reduces the cost of purchasing fresh solvents but also decreases the volume of hazardous waste, thereby minimizing environmental impact.
On the other hand, nitrogen plays a key role in reducing explosion risks. By lowering the oxygen concentration in the system below the Limit of Oxygen Concentration (LOC)—which for many VOCs ranges between 8% and 15% by volume—the likelihood of an explosion is significantly minimized. This feature makes the use of nitrogen a critical safety measure in petrochemical plants, refineries, and other high-risk industries.
Application of Nitrogen in Industrial Wastewater Treatment
Various industries typically generate wastewater containing volatile organic compounds (VOCs). Discharging such wastewater into biological treatment systems without pretreatment can disrupt microbial activity, reduce treatment efficiency, and increase VOC emissions into the environment. Using nitrogen gas in wastewater pretreatment stages, such as in homogenization tanks, significantly lowers VOC concentrations and ensures stable performance of biological treatment units.
Although air is generally a cheaper stripping gas, nitrogen is the preferred choice when VOCs are highly toxic, flammable, or oxidation-sensitive. For example, in refinery wastewater treatment where benzene is a primary contaminant, nitrogen stripping prior to biological treatment reduces explosion risks and also enables the recovery of benzene from the gas streams.
After stripping, the mixture of nitrogen and separated VOCs is typically directed to various units for VOC recovery or disposal. These units include cryogenic condensers for light compounds, activated carbon adsorption beds, and compression and distillation systems, which can achieve VOC removal efficiencies above 95% and help meet environmental standards.
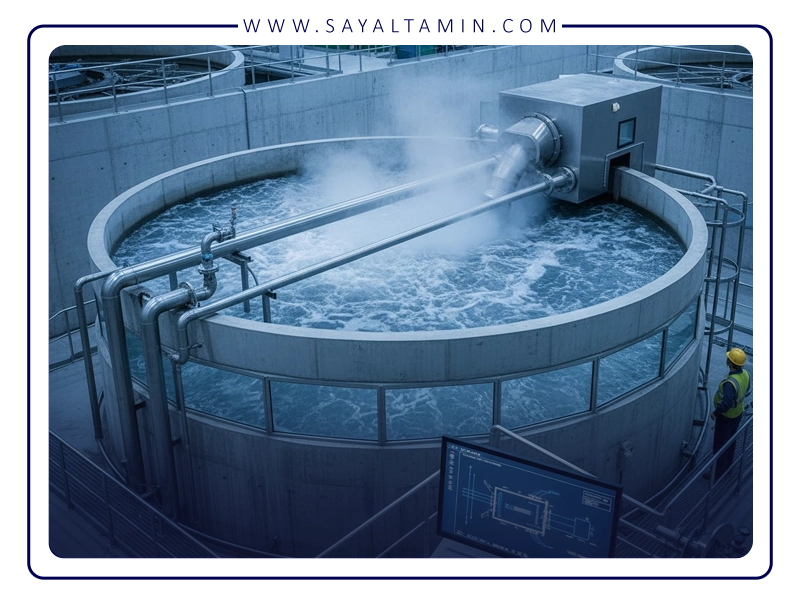
Process Design Considerations
Nitrogen stripping systems can be implemented using various types of equipment, each with its own advantages and limitations. Packed towers offer a high surface area and are suitable for continuous operations with large flow rates. Tray columns are effective for stage-wise contact and high-volume streams. Sparged tanks used in batch operations are simpler and more cost-effective but generally provide lower mass transfer efficiency.
In design, parameters such as the gas-to-liquid ratio, temperature, pressure, and contact time must be carefully optimized. Increasing the gas-to-liquid ratio enhances mass transfer efficiency but raises nitrogen consumption. Higher temperatures increase the volatility of compounds but also increase energy costs. Lower pressures can, in some cases, improve the volatility of high-boiling compounds. Adequate contact time is crucial to achieve equilibrium between the two phases. The purity of nitrogen is also application-dependent. For the removal of sensitive VOCs or to prevent explosions, nitrogen with a purity above 99.9% is required, whereas for less critical applications, 95% purity may be sufficient.
Environmental and Safety Implications
The use of nitrogen gas in VOC separation can significantly reduce the release of harmful compounds into the environment, helping industries comply with stringent environmental regulations. With removal efficiencies exceeding 95%, air pollution is minimized, protecting public health.
At the same time, as an inert gas, nitrogen reduces explosion risks and equipment corrosion by displacing oxygen. However, the hazards associated with unintended nitrogen release must be considered; in confined spaces, it can displace oxygen and cause asphyxiation. Therefore, proper ventilation systems, oxygen sensors, and safety training for personnel are essential.
Recovering VOCs from nitrogen gas streams also helps reduce the volume of hazardous waste and conserve natural resources, representing an important step toward sustainable production and a circular economy.

Case Studies
In a large refinery in the Middle East, the implementation of a nitrogen stripping system to remove benzene from industrial wastewater reduced the contaminant concentration from 45 mg/L to less than 1 mg/L. The separated benzene was recovered in condensers and returned to the production line, resulting in annual savings of over $250,000 for the refinery while also meeting stringent environmental standards.
In the European pharmaceutical industry, nitrogen was used to separate methylene chloride from reaction wastewater in the production of an oxygen-sensitive drug compound. The vapors stripped with nitrogen were collected, treated, and reused in a controlled manner, resulting in a 40% reduction in solvent costs and preventing oxidation of the active ingredient.
Economic Considerations
Although supplying nitrogen—especially at high purity—incurs costs, these are offset by significant economic benefits. Key advantages include the value of recovered VOCs, avoidance of environmental fines, reduced insurance costs due to improved safety, and extended equipment lifespan. In many industrial units that already produce nitrogen for other purposes, the marginal cost of using it for stripping is minimal.
Future Developments
Emerging technologies, such as membrane contactors, enable VOC removal with much lower nitrogen consumption and more compact designs. Additionally, hybrid systems that combine nitrogen stripping with advanced adsorption or catalytic oxidation methods can provide more effective control of complex, multi-component VOCs in the future.

Nitrogen gas serves as a safe, effective, and versatile tool in the separation of volatile organic compounds from industrial streams and wastewater. By removing oxygen from the system, it not only enhances safety but also prevents the oxidation of sensitive products, ensuring production quality. Integrating this technology with VOC recovery systems transforms separation into an opportunity for resource recycling. With tightening environmental regulations and increasing focus on sustainable production, the use of nitrogen in VOC management is becoming ever more important, leading to safer, cleaner, and more efficient industrial processes.
————————————————–
References
• Seader, J. D., Henley, E. J., & Roper, D. K. (2011). Separation Process Principles (3rd ed.). John Wiley & Sons.
• Perry, R. H., Green, D. W., & Maloney, J. O. (1997). Perry’s Chemical Engineers’ Handbook (7th ed.). McGraw-Hill.
• U.S. Environmental Protection Agency (EPA). (2020). Control Techniques for Volatile Organic Compound Emissions from Industrial Sources.
• Smith, R. (2005). Chemical Process: Design and Integration. John Wiley & Sons.
• Matsumura, M., & Tokumura, S. (2017). Nitrogen Stripping of Volatile Organic Compounds in Wastewater Treatment, Journal of Environmental Chemical Engineering, 5(3), 2445-2452.
• Joshi, A., & Satterfield, C. N. (2014). Gas Stripping and Absorption in Chemical Engineering, Separation and Purification Reviews, 43(1), 1-25.
• ASTM International. (2018). Standard Guide for Gas Stripping of Volatile Organic Compounds from Liquids (ASTM D6522








