This post is also available in: Persian Armenian
Argon, with the chemical symbol Ar and atomic number 18, is a noble gas that is colorless, odorless, nontoxic, and completely chemically inactive. These properties make argon a safe and effective choice for controlled environments. Argon makes up about 0.93% of the Earth’s atmosphere, which is more than twice the average amount of water vapor in the air. The main reason argon is so unreactive is its complete electron shell, which makes it very valuable in processes where any chemical reaction must be avoided.
Pharmaceutical Argon: Standards and Supply
Purity and compliance with standards
Pharmaceutical argon must be of very high purity (typically 99.999% or greater) to avoid any contamination that may threaten the stability or efficacy of the drug. The gas must comply with pharmaceutical standards including USP, EP, JP as well as ISO 8573-1 and GMP (Good Manufacturing Practice) principles.
Forms of supply and maintenance
Argon is available in various forms: as a gas in pressurized cylinders, as a liquid in large drums or bulk tanks, and in special packaging for smaller applications. Authorized suppliers carry out filling, transportation, storage, and quality control testing processes according to approved guidelines to provide the necessary assurance of traceability, compliance, and quality.

Main applications in pharmaceutical production
Creating an inert environment in synthesis and final packaging
During the synthesis and filling stages of a drug in a vial or ampoule, argon is used to remove oxygen and moisture from the headspace of the product to prevent oxidation and degradation of active pharmaceutical ingredients.
Sterile processes and clean environments
In sterile manufacturing processes, the use of argon helps maintain a low-oxygen, particle-free environment. This gas is widely used in isolators and clean rooms where sensitive pharmaceuticals are manufactured.
Cryogenic storage and tissue freezing
Liquid argon, with its very low boiling point, is used to freeze cells, tissues, vaccines, and biopharmaceuticals. Its inertness and low temperature preserve molecular structure and cellular viability during long-term storage.
Chromatographic and elemental analyses
In quality control laboratories, argon is used as a carrier gas or plasma gas in techniques such as ICP-MS, atomic emission spectroscopy, and gas chromatography. It is very effective in generating plasma for the analysis of heavy metals and pharmaceutical impurities.
Used in packaging, storage and product lifespan control
In pharmaceutical packaging (particularly modified atmosphere packaging – MAP), replacing the air inside the package with argon reduces oxygen, inhibits microbial growth, and increases product shelf life. Because of argon’s higher density than nitrogen, it is preferred in cases where more precise layering and better coverage are required.
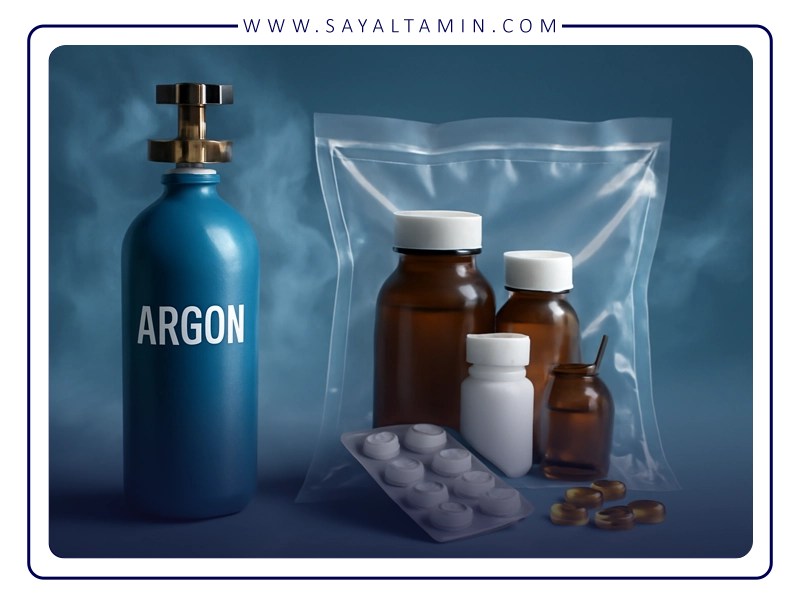
Using argon in packaging, storage, and shelf life control of pharmaceutical products
In the pharmaceutical industry, the packaging process is not simply about enclosing the product, but is a critical step in maintaining the stability, sterility, efficacy, and safety of the product throughout its shelf life. Many drug formulations, especially biologicals, sterile injectables, and some oral dosage forms, are highly sensitive to oxygen, moisture, light, and microbial contamination. To address these challenges, the use of argon gas in packaging and storage strategies has increasingly gained attention. Argon’s completely neutral nature, high density, and low solubility make it an ideal choice for protecting drugs from degradation and extending their shelf life under standard or challenging storage conditions.
| Read more: Liquid Argon in Nuclear Reactors |
Headspace degassing and oxygen replacement
One of the primary uses of argon in pharmaceutical packaging is its ability to displace oxygen from the headspace of vials, ampoules, syringes, and other primary containers. This headspace, which remains after the container is filled, typically contains ambient air and is approximately 21% oxygen. Oxygen can oxidize active pharmaceutical ingredients (APIs), preservatives, or additives, thereby reducing product efficacy or producing harmful side effects. Argon is well-suited for this application because of its higher density than air and nitrogen. When injected into the container, it forms a dense, neutral layer that effectively masks the remaining gases from the air. This protective layer minimizes contact between the drug and oxygen, greatly reducing the risk of oxidation. Argon also has no chemical or biological interactions with pharmaceutical compounds due to its complete inertness.
Advanced production lines use automated argon injection systems that inject the gas into the headspace of the container during or immediately after the filling step. In some cases, vials are flushed with argon just prior to final capping to ensure that the product is sealed in a completely oxygen-free environment. This is especially critical for sterile injectables, proteins, and other oxidation-sensitive formulations.
Modified Atmosphere Packaging (MAP) for solid forms and active ingredients
Argon is also used in modified atmosphere packaging for solid pharmaceutical forms such as tablets, capsules, granules, and powders. Although these forms are less susceptible to microbial contamination than liquid formulations, they are vulnerable to oxidation and moisture absorption, which can lead to discoloration, reduced efficacy, or impaired dissolution and absorption performance.
In these applications, argon replaces the air in the package and is injected into it before final sealing. This method is very useful for moisture- or oxidation-sensitive formulations such as effervescent tablets, probiotics, and vitamins such as C and E. Due to its complete neutrality and lack of reactivity with active ingredients or excipients, argon creates a dry, oxygen-free environment and ensures the chemical and physical stability of the product over time.
Active Pharmaceutical Ingredients (APIs) that are sensitive to light, air, or moisture also benefit from storage in an argon atmosphere. When transporting or storing these materials in leak-proof drums or multi-layer bags, the interior is often flushed with argon to protect the purity and stability of the compounds during long-term storage or international shipping.
Storage of lyophilized and freeze-dried products
Freeze-drying or lyophilization is a common method for stabilizing biologics, vaccines, and injectables. After the drying process is complete, the final product is very sensitive to moisture and oxygen, and even small amounts can lead to hydrolysis or oxidation.
For this reason, lyophilized products are usually sealed in an inert atmosphere, and argon is a preferred choice for this purpose. During the final sealing step inside the lyophilization chamber, argon is injected to displace any remaining air and protect the dried product. The high density and strong masking ability of this gas prevent the ingress of oxygen or moisture during sealing.
On the other hand, the use of argon in this process allows for quality monitoring through methods such as headspace analysis. This non-invasive method verifies the accuracy of the injection and sealing operation by measuring the amount of residual oxygen or argon inside the packaged container. This data is often provided as part of the stability documentation or validation of the container closure in the pharmaceutical dossier.

Integration with modern packaging technologies
Argon’s effectiveness in protecting pharmaceutical products is enhanced when combined with very low permeability packaging materials and advanced sealing systems. Innovations such as fully sealed glass vials, elastomeric caps with protective coatings, aluminum laminate blisters, and multi-layer films provide an effective physical barrier against gas and vapor intrusion. By infusing these systems with argon, the level of protection is significantly increased.
This is particularly critical for biologic therapeutics such as monoclonal antibodies, peptide injectables, and nucleic acid-based drugs including mRNA vaccines, as these products are highly unstable outside of controlled conditions. Argon reduces the need for chemical stabilizers by preventing contact with oxidants and maintains molecular stability.
Also, in parenteral nutrition (PN) solutions and complete nutritional mixtures (TNA), bags containing the solution are increasingly filled with argon to prevent oxidation of fats and vitamins, especially vitamins A and C. By eliminating oxygen, argon prevents the development of unpleasant odors, discoloration, and loss of therapeutic quality of the product during storage.
Comparative advantages in specialized applications
Although nitrogen is used as a cheap inert gas in pharmaceutical packaging, argon has specific advantages that make it more suitable for specialized or high-risk applications. Argon’s higher density results in a more stable and durable protective layer forming in the headspace of containers. Unlike nitrogen, which readily mixes with the remaining air, argon remains as a dense layer and is more resistant to shaking or transportation.
Additionally, argon has a lower solubility in water and organic solvents than nitrogen. This is important in sensitive aqueous formulations, as even small amounts of dissolved gas can affect pH, chemical equilibrium, or dissolved oxygen levels. Argon also does not form chemical compounds or radicals under any circumstances due to its stable electronic structure, offering the highest level of chemical neutrality.
In demanding environments where the goal is to eliminate chemical stabilizers and additives, argon serves as a non-chemical alternative to maintain product stability. This facilitates the development of simpler and cleaner formulations and speeds up the pharmaceutical approval process.
Quality control and integrity in the process
Advanced pharmaceutical packaging lines are equipped with precise sensors and control systems that monitor the flow, concentration and purity of argon throughout the process. Oxygen sensors and in-line gas analyzers ensure that the neutralization process is carried out according to standard, with final oxygen levels of less than one percent. The information collected is automatically recorded in production documents, meeting GMP requirements and regulatory inspections.
Closed-loop control systems are also used to inject argon uniformly and effectively across all production batches. These systems increase productivity and reliability by reducing human error, reducing gas consumption, and standardizing quality on a global level.
Application in the production of medical equipment
Lasers and Plasma Technologies
Argon-fluoride lasers are used in micromachining, medical device manufacturing, and photolithography technology.
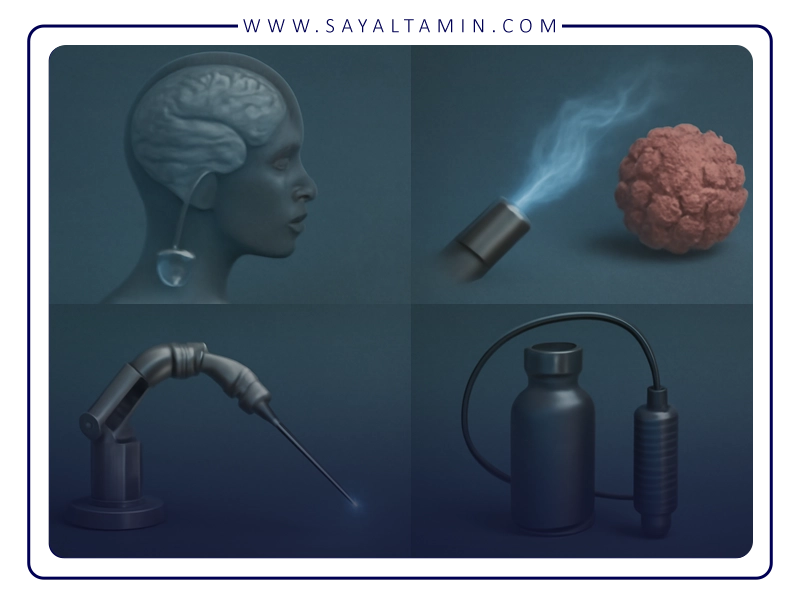
The therapeutic and procedural role of argon in medicine
Argon Plasma Coagulation (APC)
This non-contact surgical technique uses ionized argon to coagulate bleeding tissues in gastrointestinal surgeries. Its advantages include high precision, minimal damage to surrounding tissue, and low risk of organ wall penetration or perforation.
Cryoablation and cold surgery
Liquid argon is used in the destruction of abnormal tissues such as skin lesions, tumors, or precancerous tissues through rapid freezing.
Ophthalmology
Argon lasers are used to treat eye diseases such as diabetic retinopathy and retinal detachment. Argon gas is also injected into the vitreous cavity to create tamponade during some surgeries.
Emerging therapeutic research
Research is expanding into the neuroprotective effects of argon after stroke, as well as the use of cold argon plasmas to selectively destroy cancer cells.
| Must read: Use of Argon in the Steel Industry |
Safety and handling considerations
Although non-toxic, argon can cause asphyxiation in confined spaces due to its high density, as it replaces oxygen in an invisible and odorless manner. In industrial and hospital environments, the installation of proper ventilation equipment and oxygen sensors is mandatory. Also, in clinical applications, the risk of gas embolism must be managed in cases such as surgical use.
Advantages and limitations in the pharmaceutical field
Benefits:
• Complete inertness – prevents oxidation or moisture reactions
• High density – greater stability in packaging environments
• Ability to use in liquid form – possibility of storage at very low temperatures
• Versatility – can be used in manufacturing, treatment and testing
Limitations and alternatives:
• Higher cost than nitrogen
• Need for cryogenic infrastructure to store the liquid
• Alternative gases such as nitrogen, helium or CO₂ are more economical or suitable in some applications.
Applied examples in industry
Pharmaceutical packaging
To protect biological or oxygen-sensitive drugs, large pharmaceutical companies use argon to evacuate the space inside vials to reduce oxygen levels to less than 1%.
Analytical laboratories
In the examination of metal impurities according to the USP standard <232>The use of argon gas is common in ICP-MS techniques.
Biobanks and cryogenic storage
Vaccines and biological samples are stored in biobank centers using liquid argon at temperatures below -186 degrees Celsius.
Digestive treatments
The APC method is commonly used in the treatment of bleeding ulcers of the stomach and intestines and reduces the risk of perforation.
Future prospects and research direction
• Neuroprotective therapies: Using argon to protect brain tissue after stroke
• Plasma anticancer therapies: Using cold argon plasmas to destroy cancer cells
• Integration with endoscopy equipment: Design of portable or robotic systems with APC capability
• Hybrid analyses: combining argon gas with advanced sensors for more accurate detection of contaminants or microorganisms
Argon, although not a pharmaceutical compound, plays a vital role in many areas of pharmaceutical manufacturing and treatment. Its chemical neutrality, high purity, and ability to function in various forms (gas or liquid) make it a suitable choice for protecting drugs, performing precision surgeries, and laboratory analysis. As research advances and clinical uses expand, argon will have an even more important place in the future of the pharmaceutical and medical device industries.
————————————————–
Sources and references:
1. United States Pharmacopeia (USP) – General Chapter <1207>: Package Integrity Evaluation – Sterile Products.
https://www.usp.org
Standard reference for packaging integrity testing and the use of inert gases in pharmaceutical processes
2. ICH Guideline Q1A(R2): Stability Testing of New Drug Substances and Products
International Council for Harmonisation (ICH)
https://www.ich.org/page/quality-guidelines
Official guide for evaluating drug stability, which recommends the use of an inert atmosphere such as argon to maintain stability
3. Air Products and Chemicals Inc. – Pharmaceutical Gases Overview
https://www.airproducts.com
Specialized information about the use of argon and other inert gases in the pharmaceutical and biological industries




