This post is also available in: Persian Armenian
In recent decades, environmental crises and global climate change have attracted increasing attention from industries and environmentalists. One of the most important causes of these crises is the emission of greenhouse gases, especially carbon dioxide (CO₂)
.
This gas has accumulated in the Earth’s atmosphere, mainly due to human activities such as burning fossil fuels, heavy industry, and transportation, and plays a major role in global warming. In this regard, attention is increasing day by day on technologies that can capture carbon dioxide and convert it into useful resources. One of these technologies is the production of methanol (CH₃OH) from CO₂, which can play an important role in reducing greenhouse gas emissions and also in the production of basic chemicals. This method is considered not only as a green and sustainable solution but also as an economic opportunity to convert waste into valuable products.
In this article, we will examine the applications of carbon dioxide in the methanol production process, the benefits, challenges, and future of this technology.
The process of producing methanol from carbon dioxide
1. History and importance
Methanol, also known as wood alcohol, is an important raw material in the chemical industry. It is used in the production of plastics, resins, solvents, fuels, and detergents. Although traditional methods of methanol production are based on thermal processes and synthesis from lignite and coal, in recent years there has been an increasing focus on its production from alternative carbon sources.
2. Main processes
Methanol production from CO₂ is mainly carried out using processes based on specific catalysts, which include the following steps:
• Carbon dioxide capture: CO₂ is captured from various sources (such as industrial emissions, pollutant emissions, or even from the atmosphere).
• Converting CO₂ into intermediates: In the initial processes, CO₂ must be converted into a substance that can play a role in subsequent reactions, such as hydrogen.
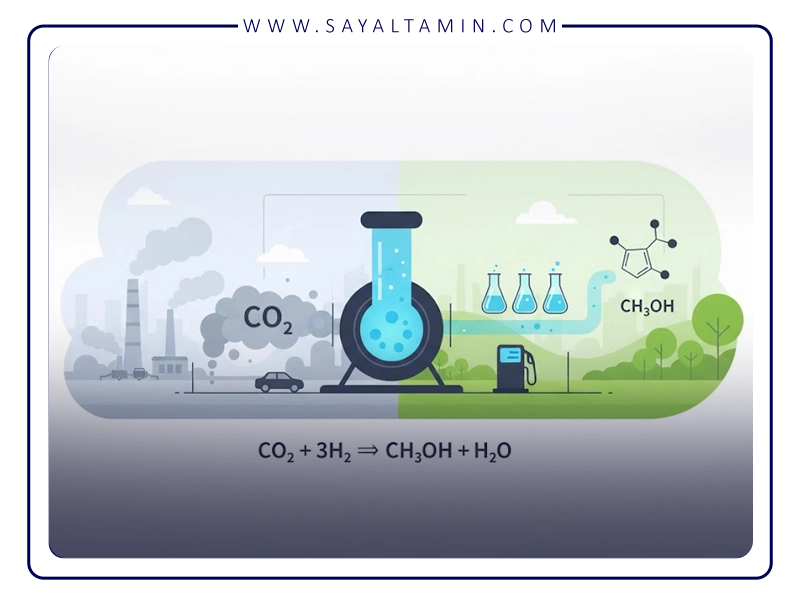
• Reaction with hydrogen: Carbon dioxide is converted to methanol with hydrogen in the presence of a catalyst. The main reaction is as follows:
CO2+3H2 →CH3OH+H2O
This reaction takes place at relatively high temperatures and at appropriate pressures.
| Read more: Use of liquid carbon dioxide (CO2) in the oil industry |
3. Hydrogen sources
One of the major challenges in producing methanol from CO₂ is the supply of hydrogen. Hydrogen must be produced from sustainable sources to ensure that the overall process remains green. There are several methods for producing hydrogen:
• Electrolysis of water with renewable energy: The best solution for producing green hydrogen with minimal emissions.
• Natural gas or coal but with cleaner, low-carbon processes.
Benefits of producing methanol from carbon dioxide
1. Reducing carbon dioxide emissions
One of the biggest benefits of this technology is that it helps reduce the amount of CO₂ in the atmosphere. By capturing and converting CO₂ from industrial sources and even from the atmosphere, greenhouse gas emissions can be reduced.
2. Reuse of waste and non-renewable resources
This process has great potential as a solution for recycling carbon waste and converting it into a valuable material. As a result, mineral and fossil resources are reduced and dependence on fossil fuels is reduced.
3. Sustainable and economical chemical production
Methanol produced from CO₂ could replace traditional methanol in various industries. This strategy, if successfully commercialized, could contribute to a green and sustainable economy and create new job opportunities.
4. Easy storage and transportation
Methanol, unlike crude gases, can be stored and transported, and can be used as an energy carrier and fuel source. This property presents an opportunity for the development of sustainable supply chains and the transition to renewable energy.
Challenges and limitations of the methanol production process from CO₂
Every emerging and innovative technology faces challenges that must be overcome:
1. High costs of providing green hydrogen
In recent years, the cost of producing green hydrogen through innovative methods, such as electrolysis, has decreased, but it is still relatively expensive, which could be an obstacle to the economicization of this technology.
2. The need for stable and high-yield catalysts
In the process of converting CO₂ to methanol, the efficiency and selectivity of the reactions are of great importance. Developing stable, robust and efficient catalysts is one of the challenges for researchers.
3. Energy required for chemical processes
Most CO₂ conversion processes require significant energy, and if non-renewable resources are used, the technology may not have a green conclusion. Harnessing solar and wind energy is key to solving this problem.
| Must Read: How to Supply and Deliver Carbon Dioxide (CO2) |
4. The issue of CO₂ storage and transport
If CO₂ is captured from natural or industrial sources, safe and economical technologies are needed to store and transport it. It is also important to prevent leaks and emissions from these processes.

Futures Studies and Development Horizons
With technological advances in catalysts, green hydrogen sources, and the exploitation of renewable energies, the outlook for methanol production from CO₂ is very positive.
1. Development of electrolysis technologies
Increasing the efficiency and reducing the costs of water electrolysis plays an important role in building a green and economic value chain.
2. Policy strategies
Support from governments and policymakers in the development of low-carbon technologies can play a key role in accelerating these processes.
3. International collaborations
Collaboration between researchers, industries, and governments is essential to develop shared technologies, reduce costs, and accelerate commercialization.
4. Research and innovation in catalysts
Advances in the field of efficient and robust catalysts can increase reaction rates and reduce production costs.
As a result, the process of producing methanol from carbon dioxide is a great opportunity for the development of green technologies and reducing the negative impacts of human activities on the environment. As a new solution, this technology can not only be effective in reducing the amount of carbon dioxide in the atmosphere, but also play an important role in future economies based on renewable energy.
Although challenges such as the high cost of hydrogen and catalytic technologies still remain, with technological advancements, support, and international cooperation, a sustainable and green future awaits. A future in which carbon waste becomes valuable and useful, and humanity can achieve economic and industrial development with minimal damage to the environment.
————————————————–
References
1. Carbon Dioxide Utilization for the Production of Chemicals and Fuels
2. Methanol: The Basic Chemical and Energy Feedstock of the Future
3. Catalytic Carbon Dioxide Conversion
4. Green Carbon Dioxide: Advances in CO₂ Utilization
5. Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass
6. www.sciencedirect.com
7. www.frontiersin.org




